
I bet a lot of you have
consumed at least one thing that has vanilla as its flavouring. Vanilla
ice-cream, cake, vanilla syrup in your ice coffee, French vanilla latte,
vanilla flavoured lotions, etc. It is such a common flavour used excessively in
a lot of industries. Personally, I used to think it was only used in food
products, but after some research on the compound 'Vanillin' (the chemical
comprising vanilla) I learnt that it can be more than a flavouring compound. It
is a source of L-dopa, which is a very important drug used in the treatment of
Parkinson's disease (4). It can also be used as a starting material for production
of methyldopa, another important drug that is commonly used to lower blood
pressure (7). It is also widely used as a preservative and has shown to increase
shelf life upto 60 days (3). Furthermore, it has some industrial uses and used in
insecticides to attract insects due to its sweet smell.
Its
consumption in humans is safe (the most dangerous effect being eye irritations), hence it is still being used in production of
food products, cosmetics and drugs (5). Some people can be allergic to Vanillin (1). Some studies showed that small doses of vanillin can be lethal in mouse and
rats if inhaled (6).
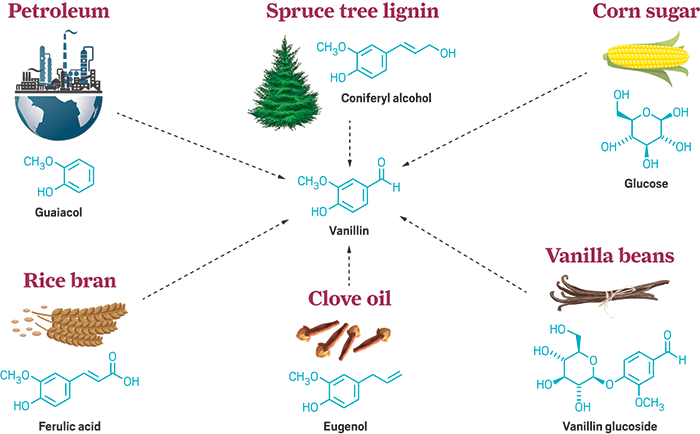
Despite
its many uses, the availability of organic vanilla is scarce: vanilla bean or
ferulic acid in rice and oats. Although there are many other sources that can
be used to produce artificial vanilla. These are available from cloves in the
form of eugenol oil, ylang ylang plant (tropical plant) that produces
isoeugenol, and turmeric with curcumin extracts (2). These extracts can undergo
chemical processes to give synthetic vanillin and hence, synthetic vanilla
extracts (2). You must have noticed that all these extracts whether organic or
inorganic come from plants. Thus, a huge controversy surrounding vanillin is in
regards to "What's 'natural' and what's 'artificial'".
References:
1. Bingham, E., Cohrssen, B., & Powell, C.H.
(2001). Patty's Toxicology (5th ed.). New York, NY: John
Wiley & Sons.
2. Burdock,
G.A. (2001). Fenaroli's Handbook of
Flavor Ingredients (6th ed.). Boca Raton, FL
3. Cerrutti
P et al. (1997). J of Food Science, 62(3),
608-610.
4. Lewis,
R.J. Sr. (2007). Hawley's Condensed
Chemical Dictionary (15th ed.). Inc. New York, NY: John Wiley & Sons.
5. Lewis,
R.J. (1996). Sax's Dangerous Properties
of Industrial Materials (9th ed.). New York, NY: Van Nostrand Reinhold
6. Tamai K
et al. (1992). Mutat Res, 268(2),
231-7.
7. Vidal J.P.
(2006) Vanillin. Kirk-Othmer Encyclopedia of Chemical Technology (1999-2015).
John Wiley & Sons, Inc.
No comments:
Post a Comment